Lab: Types of Chemical Reactions
Student Guide
This laboratory allows you to study various ki...

Lab: Types of Chemical Reactions
Student Guide
This laboratory allows you to study various kinds of chemical reactions, including some that result in precipitates.
Lesson Objectives
• Compare and contrast synthesis, single-displacement, and double-displacement reactions.
PREPARE
Approximate lesson time is 60 minutes.
Materials
• Lab Instructions: Lab_5.08_Instructions_modified_2020
• Lab Report: Lab_5.08_Report_modified_2020
• Lab Guidelines: Lab_Guidelines_modified
LEARN
Activity 1: Types of Chemical Reactions 1
Instructions
As you read through the lesson online, use the space below to take notes.
In this laboratory, you will study different kinds of chemical reactions.
Knowing the types of reactions helps you interpret your observations.
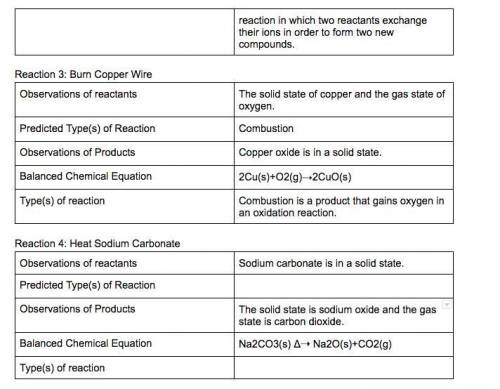
In a synthesis reaction, two reactants unite to form a third product.
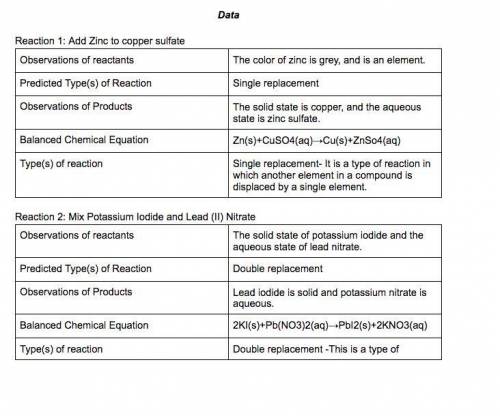
In a single-displacement reaction, one ion of a reactant bonds with the second reactant.
In a double-displacement reaction, ions of both reactants change places.
Activity 2: Types of Chemical Reactions 1
Instructions
Procedure
1. Open the Chemical Reactions Virtual Lab.
2. Click View the Tutorial and complete the tutorial to learn how to conduct the lab.
3. Close the tutorial and click begin the Lab.
Part 1 Synthesis Reaction
4. Perform the procedure, placing the magnesium strip in the flame.
5. Record your reaction.
6. Research the chemical reaction of magnesium and oxygen gas. Write an equation for the chemical reaction
that accounts for the observed reaction in this part of the lab.
7. Answer the question: What is a synthesis reaction?
8. Answer the questions on Part 1 in the Lab Report.
Part 2 Single Displacement Reaction
9. Place 1 scoop of zinc in Vial A and add 10 drops of copper (II) sulfate. Observe the reaction.
10. Place ball of aluminum in Vial B and add 10 drops of copper (II) sulfate. Observe the reaction.
11. Place 1 scoop of zinc in Vial C and add 10 drops of silver nitrate. Observe the reaction.
12. Place copper wire in Vial D and add 10 drops of silver nitrate, wait 5 minutes. Observe the reaction.
13. Complete the ta

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 22:00
For a family dinner, jose’s mom baked a loaf of bread. during the meal, his grandmother commented, “this bread is so dense! ” what do you think his grandmother meant by the word dense? you have some ideas regarding density already, but this experiment will enrich your understanding of the relationship between mass, volume and the density of objects. when making measurements in this experiment we will be using si units, the international system of units. the purpose of using si units is to have the ability to communicate and compare data results with other experiments without having to make conversions. measurement symbol si unit length m meter mass kg kilogram volume l liters density kg/l kilograms per liter any calculations that are performed during this experiment will ultimately be reported in scientific notation. recall that scientific notation is extremely important when we have data that is extremely small or large. scientists rely on a standard form of communication to quickly make hypotheses and judgements based on data. what is the density of block a? a0kg/l what is the density of block b? a1kg/l what is the density of block c? a2kg/l what is the density of block d? a3kg/l what is the density of block e? a4kg/l which block is the densest? a5
Answers: 1
You know the right answer?
Questions











Computers and Technology, 27.07.2021 17:50


Mathematics, 27.07.2021 17:50

Chemistry, 27.07.2021 17:50


Computers and Technology, 27.07.2021 17:50

Mathematics, 27.07.2021 17:50


English, 27.07.2021 17:50

Mathematics, 27.07.2021 17:50