

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 23.06.2019 05:30
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1
You know the right answer?
Calculate the pH at 25 degrees celsius of a 0.39 M solution of pyridinium chloride (c5h5nhcl) . Note...
Questions
















Computers and Technology, 08.10.2019 00:10




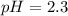
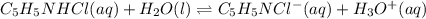
![Ka=\frac{[C_5H_5NCl^-][H_3O^+]}{[C_5H_5NHCl]}](/tpl/images/0880/2037/d1e08.png)
 , we write:
, we write: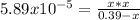
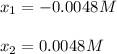
![pH=-log([H_3O^+])=-log(0.0048)\\\\pH=2.3](/tpl/images/0880/2037/04092.png)


