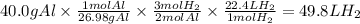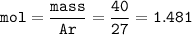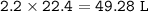Chemistry, 06.11.2020 04:20 crawford184232323234
What is the volume in liters of hydrogen gas that would be produced by the reaction of 40.0 g of Al with excess HCl at STP according to the following reaction? 2 Al (s) + 6 HCl (aq) → 2 AlCl₃ (aq) + 3 H₂ (g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2
You know the right answer?
What is the volume in liters of hydrogen gas that would be produced by the reaction of 40.0 g of Al...
Questions






Business, 27.11.2020 21:50


Mathematics, 27.11.2020 21:50








Computers and Technology, 27.11.2020 21:50