
Physics, 12.08.2020 04:01 scavalieri3746
Warm blooded animals are homeothermic; that is, they maintain an approximately constant body temperature. (Forhumans it's about 37 oC.) When they are in an environment that is below their optimum temperature, they use energy derived from chemical reactions within their bodies to warm them up. One of the ways that animals lose energy to their environment is through radiation. Every object emits electromagnetic radiation that depends on its temperature. For very hot objects like the sun, that radiation is visible light. For cooler objects, like a house or a person, that radiation is in the infrared and is invisible. Nonetheless, it still carries energy. Other ways that energy is lost by a warm animal to a cool environment includes conduction (direct touching of a cooler object) and convection (cooler air moving and carrying thermal energy away). See Heat Transfer for a discussion of all three.
For this problem, we'll just consider how much energy an animal needs to burn (obtain from internal chemical reactions) in order to stay warm just from radiation losses. The rate at which an object loses energy through radiation is given by the Stefan-Boltzmann equation:
Rate of energy loss = AεσT4
where T is the absolute (Kelvin) temperature, A is the area of the object, ε is the emissivity (unitless and =1 for a perfect emitter, less for anything else), and σ is the Stefan-Boltzmann constant:
σ = 5.67 x 10-8 J/(s m2 K4)
Consider a patient trying to sleep naked in a cool room (55 oF = 13 oC). Assume that the person being considered is a perfect emitter and absorber of radiation (ε = 1), has a surface area of about 2.5 m2, and a mass of 80 kg.
a. A person emits thermal radiation at a rate corresponding to a temperature of 37 oC and absorbs radiation at a rate (from the air and walls) corresponding to a temperature of 13 oC. Calculate the individual's net rate of energy loss due to radiation (in Watts = Joules/second).
net rate of energy loss = Watts
b. Assume the patient produces no energy to keep warm. If they have a specific heat about equal to that of water (1 Cal/kg-oC) how much would their temperature fall in one hour? (1 Cal = 1kcal = 103 cal)
ΔT = oC
c. Given that the energy density of fat is about 9 Cal/g, how many grams of fat would the person have to utilize to maintain their body temperature in that environment for one hour?
amount of fat needed = g

Answers: 2
Another question on Physics

Physics, 21.06.2019 19:30
11. you want to calculate the displacement of an object thrown over a bridge. using -10 m/s2 for acceleration due to gravity, what would be the total displacement of the object if it took 8 seconds before hitting the water?
Answers: 1

Physics, 21.06.2019 23:00
The key to stability is feedback between the reservoir and the fluxes into and/or out of the reservoir. assume that the rate of outflow from a reservoir depends on the size of the reservoir according to the following relationship: outflow rate= k x (size of reservoir), where k is a constant. a reservoir of water has a volume of 5000 liters, and the rate of outflow at steady state is 25 liters per minute. what is k? (give both the numerical value and its units.) what is the residence time? what is the relationship between k and the residence time?
Answers: 1


You know the right answer?
Warm blooded animals are homeothermic; that is, they maintain an approximately constant body tempera...
Questions










Health, 24.02.2020 18:02


Computers and Technology, 24.02.2020 18:02

Mathematics, 24.02.2020 18:02



Computers and Technology, 24.02.2020 18:02

English, 24.02.2020 18:02



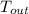 = 37 + 273 = 310 K
= 37 + 273 = 310 K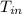 = 13 + 273 = 286 K
= 13 + 273 = 286 K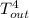 -
- 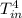 )
)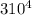 -
- 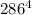 ) = 360.7 J/s
) = 360.7 J/s

