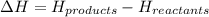Chemistry, 30.07.2019 09:20 lilyrockstarmag
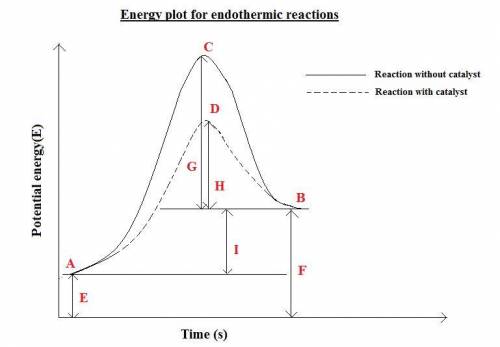
How is enthalpy used to predict whether a reaction is endothermic or exothermic? when the enthalpy of the reactants is higher than the enthalpy of the products, the reaction is endothermic. when the enthalpy of the products is higher than the enthalpy of the reactants, the reaction is exothermic. when the enthalpy change of the reaction is positive, the reaction is exothermic. when the enthalpy change of the reaction is positive, the reaction is endothermic.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
How is enthalpy used to predict whether a reaction is endothermic or exothermic? when the enthalpy...
Questions

History, 28.07.2019 21:50

Biology, 28.07.2019 21:50

Mathematics, 28.07.2019 21:50

History, 28.07.2019 21:50


Social Studies, 28.07.2019 21:50


Social Studies, 28.07.2019 22:00


History, 28.07.2019 22:00

History, 28.07.2019 22:00

History, 28.07.2019 22:00

History, 28.07.2019 22:00

History, 28.07.2019 22:00

History, 28.07.2019 22:00

History, 28.07.2019 22:00

Physics, 28.07.2019 22:00



Physics, 28.07.2019 22:00

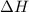 for the reaction comes out to be negative.
for the reaction comes out to be negative.