
Chemistry, 03.10.2021 01:00 memoryofdale
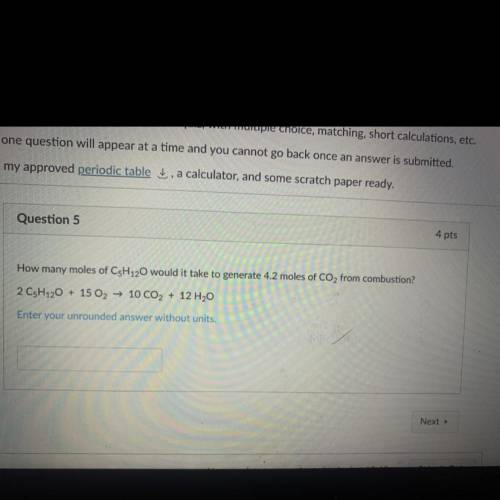
How many moles of C5H120 would it take to generate 4.2 moles of CO2 from combustion?
2 CsH120 + 15 O2 → 10 CO2 + 12 H2O
Enter your unrounded answer without units.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2

You know the right answer?
How many moles of C5H120 would it take to generate 4.2 moles of CO2 from combustion?
2 CsH120 + 15...
Questions


Social Studies, 19.03.2020 08:20


History, 19.03.2020 08:20



Mathematics, 19.03.2020 08:21




Social Studies, 19.03.2020 08:21



Mathematics, 19.03.2020 08:21




Advanced Placement (AP), 19.03.2020 08:22




