Chemistry, 11.06.2021 22:50 tyneshiajones124
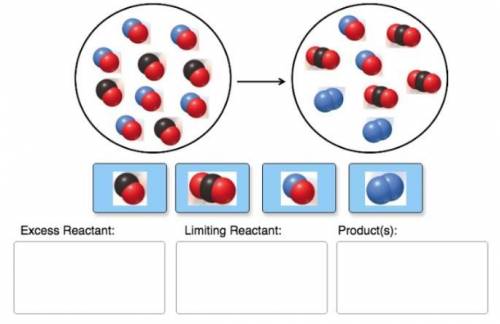
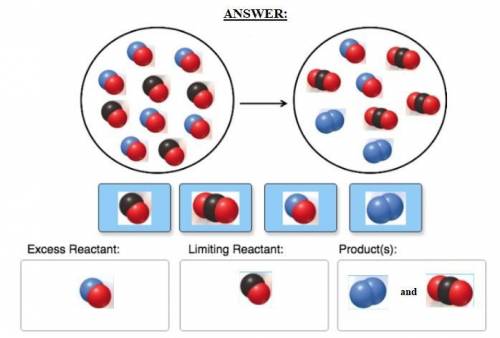
Consider the reaction of NO and CO to form N2 and CO2, according to the balanced equation: 2 NO (g) + 2 CO (g) → N2 (g) + 2 CO2 (g) Identify the excess reactant, the limiting reactant, and the product(s) using the molecular art. (Black spheres are carbon, blue spheres are nitrogen, and red spheres are oxygen.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

Chemistry, 23.06.2019 08:00
How does the digestive system interact with the circulatory system? a. messages sent as electrical impulses from the digestive system are transported throughout the body by the circulatory system. b. nutrients taken in and broken down by the digestive system are carried to various parts of the body by the circulatory system. c. nutrients and gases are absorbed by organs in the circulatory system. then, they are transported to all parts of the body by organs in the digestive system. d. oxygen and carbon dioxide are exchanged by organs in the digestive system, and the gases are carried to the rest of the body by the circulatory system.
Answers: 2
You know the right answer?
Consider the reaction of NO and CO to form N2 and CO2, according to the balanced equation: 2 NO (g)...
Questions



Biology, 02.07.2019 17:20


Biology, 02.07.2019 17:20

Biology, 02.07.2019 17:20

Biology, 02.07.2019 17:20

Biology, 02.07.2019 17:20

Biology, 02.07.2019 17:20


Biology, 02.07.2019 17:20



Biology, 02.07.2019 17:20



Mathematics, 02.07.2019 17:30



History, 02.07.2019 17:30

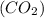 compound
compound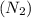
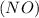 compound
compound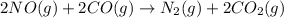
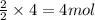 of NO
of NO