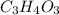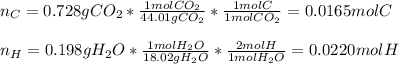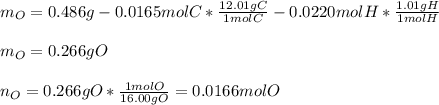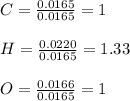Chemistry, 31.05.2021 15:30 bermudezs732
Vitamin C has the formula CxHyOz. You burn 0.486 g of the compound in a combustion analysis chamber and isolate 0.728 g of CO2 and 0.198 g of H2O. What is the empirical formula? Enter the elements in the order C, H, and O.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
Vitamin C has the formula CxHyOz. You burn 0.486 g of the compound in a combustion analysis chamber...
Questions


English, 13.11.2019 23:31

Mathematics, 13.11.2019 23:31


Mathematics, 13.11.2019 23:31

Mathematics, 13.11.2019 23:31


Geography, 13.11.2019 23:31


Social Studies, 13.11.2019 23:31


English, 13.11.2019 23:31

Health, 13.11.2019 23:31

Mathematics, 13.11.2019 23:31


Social Studies, 13.11.2019 23:31


Business, 13.11.2019 23:31

History, 13.11.2019 23:31

Mathematics, 13.11.2019 23:31