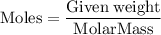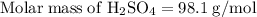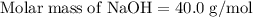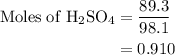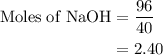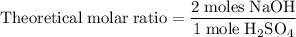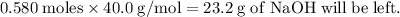Chemistry, 06.10.2019 19:30 lilbrown6369
Aqueous sulfuric acid h2so4 will react with solid sodium hydroxide naoh to produce aqueous sodium sulfate na2so4 and liquid water h2o . suppose 89.3 g of sulfuric acid is mixed with 96. g of sodium hydroxide. calculate the minimum mass of sulfuric acid that could be left over by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
Aqueous sulfuric acid h2so4 will react with solid sodium hydroxide naoh to produce aqueous sodium su...
Questions

Mathematics, 30.01.2020 13:44

English, 30.01.2020 13:44


Mathematics, 30.01.2020 13:44

Biology, 30.01.2020 13:44



Arts, 30.01.2020 13:44

Mathematics, 30.01.2020 13:44


English, 30.01.2020 13:44

English, 30.01.2020 13:44




History, 30.01.2020 13:44

Mathematics, 30.01.2020 13:44

Chemistry, 30.01.2020 13:44


English, 30.01.2020 13:44

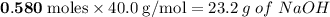 will be left.
will be left.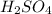 will react with solid sodium hydroxide NaOH to produce aqueous sodium sulfate
will react with solid sodium hydroxide NaOH to produce aqueous sodium sulfate 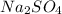 and liquid water
and liquid water 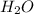 .
.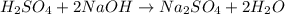
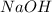 into moles by using the formula,
into moles by using the formula,