Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
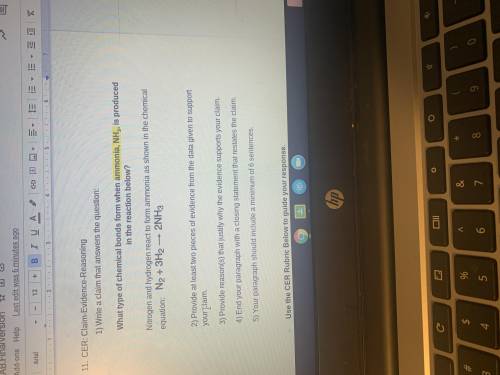
What type of chemical bonds form when ammonia, NH3, is produced in the reaction N2+3H2—>2NH3
Questions

English, 30.10.2021 23:20


Social Studies, 30.10.2021 23:20

Biology, 30.10.2021 23:20




Chemistry, 30.10.2021 23:20

German, 30.10.2021 23:20


English, 30.10.2021 23:20





Mathematics, 30.10.2021 23:30

Mathematics, 30.10.2021 23:30