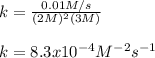Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1
You know the right answer?
What is the rate constant of a reaction if rate = 1 x 10^-2 (mol/L)/s, [A] is 2 M,
[B] is 3 M, m =...
Questions

Physics, 09.10.2019 22:00



Mathematics, 09.10.2019 22:00




Mathematics, 09.10.2019 22:00



Mathematics, 09.10.2019 22:00



Computers and Technology, 09.10.2019 22:00






![k=\frac{r}{[A]^2[B]}](/tpl/images/1150/6049/0baf7.png)