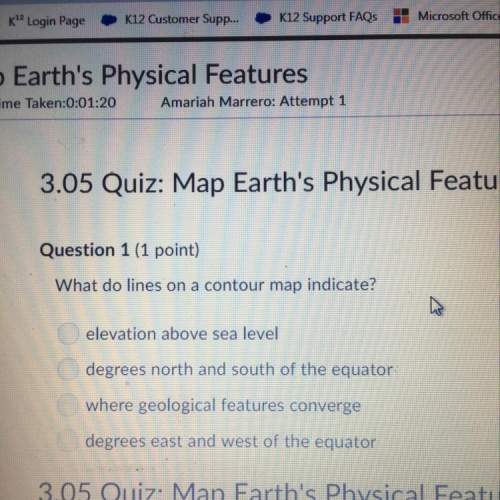
4. How much water would be needed to dilute at 750 ml solution of 2.8M HCl to a 1.0 M solution?
5. If 550 mL of a 3.50 M KCl solution is left out to evaporate until the volume is 275 mL, what would the
new Molarity be?
6. Extra Credit: A solution of H, SO, has a molarity of 0.14 mol/L in 750 mL. If 250 mL of water is added:
a. What is the new molarity of the solution?
Three questions in 1 less see how good really is
Please help I am being timed

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
4. How much water would be needed to dilute at 750 ml solution of 2.8M HCl to a 1.0 M solution?
5....
Questions

Mathematics, 04.03.2020 13:53



Chemistry, 04.03.2020 13:53

Health, 04.03.2020 13:54



Mathematics, 04.03.2020 13:56

Biology, 04.03.2020 13:56

Spanish, 04.03.2020 13:57

Social Studies, 04.03.2020 13:57

Health, 04.03.2020 14:03




English, 04.03.2020 14:17

Arts, 04.03.2020 14:30

English, 04.03.2020 14:31

English, 04.03.2020 14:32

Physics, 04.03.2020 14:33