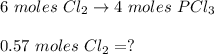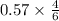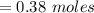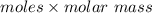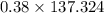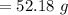Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
You know the right answer?
A 12.39 g sample of phosphorus reacts with 40.75 g of chlorine to form only phosphorus trichloride (...
Questions






Mathematics, 17.12.2021 01:00

Mathematics, 17.12.2021 01:00






Social Studies, 17.12.2021 01:00


Biology, 17.12.2021 01:00

Biology, 17.12.2021 01:00

Mathematics, 17.12.2021 01:00


Biology, 17.12.2021 01:00

Mathematics, 17.12.2021 01:00


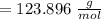
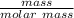
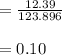
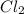 molar mass
molar mass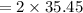
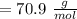
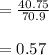
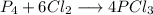
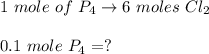
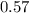 ,
, 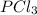 Theoretical performance
Theoretical performance