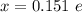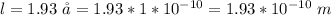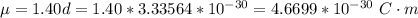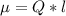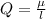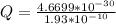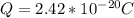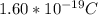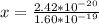Chemistry, 23.09.2020 14:01 solikhalifeoy3j1r
Determine the partial negative charge on the bromine atom in a c−br bond. the bond length is 1.93 å and the bond dipole moment is 1.40 d . express your answer using 3 significant figures. the partial negative charge on the bromine atom = previous answersrequest answer incorrect; try again; 4 attempts remaining provide feedback.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1


Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1
You know the right answer?
Determine the partial negative charge on the bromine atom in a c−br bond. the bond length is 1.93 å...
Questions

Mathematics, 25.06.2019 19:30

Mathematics, 25.06.2019 19:30

Computers and Technology, 25.06.2019 19:30




Mathematics, 25.06.2019 19:30



Physics, 25.06.2019 19:30


Biology, 25.06.2019 19:30


History, 25.06.2019 19:30



Mathematics, 25.06.2019 19:30

Mathematics, 25.06.2019 19:30

History, 25.06.2019 19:30