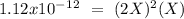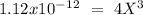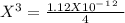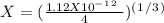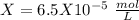Chemistry, 23.07.2020 19:01 dustonangiecook
chromate is sparingly soluble in aqueous solutions. The Ksp of Ag2CrO4 is 1.12×10−12 . What is the solubility (in mol/L) of silver chromate in 1.00 M potassium chromate aqueous solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
chromate is sparingly soluble in aqueous solutions. The Ksp of Ag2CrO4 is 1.12×10−12 . What is the s...
Questions

Mathematics, 16.02.2020 18:45


Mathematics, 16.02.2020 18:47

Mathematics, 16.02.2020 18:48


Business, 16.02.2020 18:49




Arts, 16.02.2020 18:50


Mathematics, 16.02.2020 18:51


Mathematics, 16.02.2020 18:55



English, 16.02.2020 18:57

Law, 16.02.2020 18:59

Social Studies, 16.02.2020 19:00

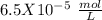
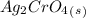 , so:
, so: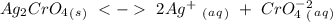
![Kps~=~[Ag^+]^2[CrO_4^-^2]](/tpl/images/0711/8854/b7f4a.png)
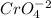 formed, 2 moles of
formed, 2 moles of 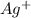 are formed. We can use "X" for the unknown concentration of each ion, so:
are formed. We can use "X" for the unknown concentration of each ion, so:![[CrO_4^-^2]~=~X](/tpl/images/0711/8854/4fd99.png) and
and ![[Ag^+]~=~2X](/tpl/images/0711/8854/da6f8.png)