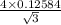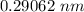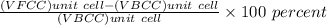Chemistry, 20.05.2020 12:58 xsanexensprocke7341
Iron (Fe) undergoes an allotropic transformation at 912°C: upon heating from a BCC (α phase) to an FCC (γ phase). Accompanying this transformation is a change in the atomic radius of Fe—from RBCC = 0.12584 nm to RFCC = 0.12894 nm. The highest density planes in BCC structure is (110) and for FCC structure is (111). i. Compare the planar density of the two. (110) in BCC and (111) in FCC iron. EA = − 1.436 r ER = 5.8 × 10−6 r 9 ii. Do you think a (111) plane in FCC structure is more amenable to dislocation motion or (110) plane in BCC structure? What is an implication of that on the mechanical properties of materials.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

You know the right answer?
Iron (Fe) undergoes an allotropic transformation at 912°C: upon heating from a BCC (α phase) to an F...
Questions

Mathematics, 23.09.2021 06:30



English, 23.09.2021 06:30

History, 23.09.2021 06:30



Biology, 23.09.2021 06:30

English, 23.09.2021 06:30





Social Studies, 23.09.2021 06:30


Mathematics, 23.09.2021 06:30


Arts, 23.09.2021 06:30


Mathematics, 23.09.2021 06:30