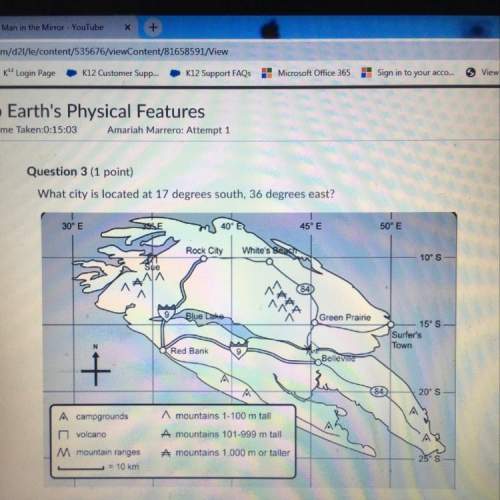
A 5.0-L flask contains a gas sample at pressure of 1.0 atm. Calculate the pressure
in the flas...

Chemistry, 05.05.2020 21:30 flyingcerberus1408
A 5.0-L flask contains a gas sample at pressure of 1.0 atm. Calculate the pressure
in the flask if the volume is doubled to 10.0 L, assuming that the temperature and amount of gas are kept constant.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1
You know the right answer?
Questions

Geography, 28.01.2021 03:30

Mathematics, 28.01.2021 03:30


Mathematics, 28.01.2021 03:30


Mathematics, 28.01.2021 03:30




Mathematics, 28.01.2021 03:30


Social Studies, 28.01.2021 03:30


Mathematics, 28.01.2021 03:30

Mathematics, 28.01.2021 03:30

Mathematics, 28.01.2021 03:30

Physics, 28.01.2021 03:30


Mathematics, 28.01.2021 03:30