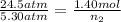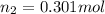A rigid tank contains 1.40 moles of an ideal gas. Determine the number of moles of gas that must be withdrawn from the tank to lower the pressure of the gas from 24.5 atm to 5.30 atm. Assume the volume of the tank and the temperature of the gas remain constant during this operation.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
You know the right answer?
A rigid tank contains 1.40 moles of an ideal gas. Determine the number of moles of gas that must be...
Questions



Mathematics, 30.09.2019 20:30


Mathematics, 30.09.2019 20:30

History, 30.09.2019 20:30

Mathematics, 30.09.2019 20:30

Computers and Technology, 30.09.2019 20:30



Computers and Technology, 30.09.2019 20:30



Mathematics, 30.09.2019 20:30


Biology, 30.09.2019 20:30

History, 30.09.2019 20:30


Biology, 30.09.2019 20:30

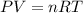
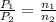
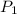 = initial pressure of gas = 24.5 atm
= initial pressure of gas = 24.5 atm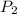 = final pressure of gas = 5.30 atm
= final pressure of gas = 5.30 atm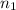 = initial number of moles of gas = 1.40 moles
= initial number of moles of gas = 1.40 moles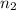 = final number of moles of gas = ?
= final number of moles of gas = ?