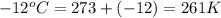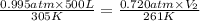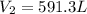Chemistry, 20.04.2020 20:05 jnsoccerboy7260
A weather balloon is filled with helium that occupies a volume of 500 L at 0.995 atm and 32.0 ℃. After it is released, it rises to a location where the pressure is 0.720 atm and the temperature is -12 ℃. What is the volume of the balloon at the new location?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
A weather balloon is filled with helium that occupies a volume of 500 L at 0.995 atm and 32.0 ℃. Aft...
Questions

Mathematics, 11.04.2020 15:04

Mathematics, 11.04.2020 15:04


Biology, 11.04.2020 15:04

History, 11.04.2020 15:04

Mathematics, 11.04.2020 15:05



Mathematics, 11.04.2020 15:05





History, 11.04.2020 15:06

Mathematics, 11.04.2020 15:06



English, 11.04.2020 15:07

Business, 11.04.2020 15:07

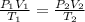
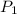 = initial pressure of gas = 0.995 atm
= initial pressure of gas = 0.995 atm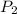 = final pressure of gas = 0.720 atm
= final pressure of gas = 0.720 atm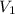 = initial volume of gas = 500 L
= initial volume of gas = 500 L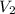 = final volume of gas = ?
= final volume of gas = ?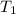 = initial temperature of gas =
= initial temperature of gas = 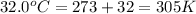
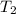 = final temperature of gas =
= final temperature of gas =