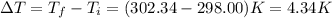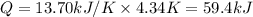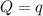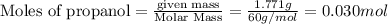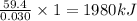Chemistry, 16.04.2020 01:46 maggie123456751
The combustion of 1.771 g of propanol ( C 3 H 7 OH ) increases the temperature of a bomb calorimeter from 298.00 K to 302.34 K . The heat capacity of the bomb calorimeter is 13.70 kJ/K . Determine Δ H for the combustion of propanol to carbon dioxide gas and liquid water.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1


Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
The combustion of 1.771 g of propanol ( C 3 H 7 OH ) increases the temperature of a bomb calorimeter...
Questions


Mathematics, 26.11.2019 03:31



Mathematics, 26.11.2019 03:31



English, 26.11.2019 03:31



Biology, 26.11.2019 03:31

Mathematics, 26.11.2019 03:31


Mathematics, 26.11.2019 03:31

Mathematics, 26.11.2019 03:31

Mathematics, 26.11.2019 03:31

Spanish, 26.11.2019 03:31



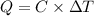
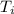 = 298.00 K
= 298.00 K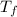 = 302.34 K
= 302.34 K