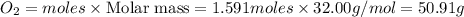Chemistry, 15.04.2020 03:54 Ashley606hernandez
Diborane (B2H6) is a gas at room temperature that forms explosive mixtures with air. It reacts with oxygen according to the following equation (which may or may not be balanced): B2H6 (g) + O2 (g) → B2O3 (s) + H2O (l) How many grams of O2 (molar mass 32.00 g/mol) will react with 14.67 grams of diborane (molar mass 27.67 g/mol). Your answer must be expressed to the correct number of significant figures, and with the correct unit.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3
You know the right answer?
Diborane (B2H6) is a gas at room temperature that forms explosive mixtures with air. It reacts with...
Questions

Computers and Technology, 07.04.2020 20:05

Computers and Technology, 07.04.2020 20:05






Biology, 07.04.2020 20:05




Advanced Placement (AP), 07.04.2020 20:06

Mathematics, 07.04.2020 20:06

Social Studies, 07.04.2020 20:06

Chemistry, 07.04.2020 20:06

English, 07.04.2020 20:06

Mathematics, 07.04.2020 20:06



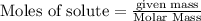
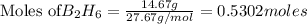
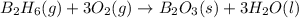
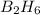 require = 3 moles of
require = 3 moles of 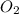
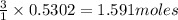 of
of