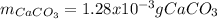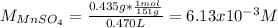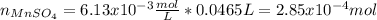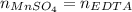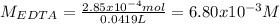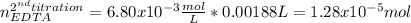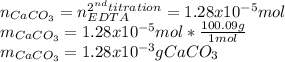Chemistry, 15.04.2020 01:15 canonmille2
A 46.50 mL aliquot from a 0.470 L solution that contains 0.435 g of MnSO 4 ( MW = 151.00 g/mol) required 41.9 mL of an EDTA solution to reach the end point in a titration. What mass, in milligrams, of CaCO 3 ( MW = 100.09 g/mol) will react with 1.88 mL of the EDTA solution?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
A 46.50 mL aliquot from a 0.470 L solution that contains 0.435 g of MnSO 4 ( MW = 151.00 g/mol) requ...
Questions


Mathematics, 26.11.2020 01:00

Biology, 26.11.2020 01:00



Computers and Technology, 26.11.2020 01:00


English, 26.11.2020 01:00

Computers and Technology, 26.11.2020 01:00


Chemistry, 26.11.2020 01:00

Social Studies, 26.11.2020 01:00


Mathematics, 26.11.2020 01:00


Computers and Technology, 26.11.2020 01:00


Chemistry, 26.11.2020 01:00