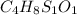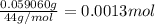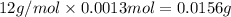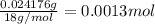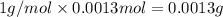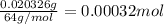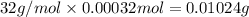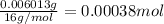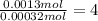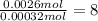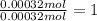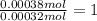A 33.153 mg sample of a chemical known to contain only carbon, hydrogen, sulfur, and oxygen is put into a combustion analysis apparatus, yielding 59.060 mg of carbon dioxide and 24.176 mg of water. In another experiment, 47.029 mg of the compound is reacted with excess oxygen to produce 20.326 mg of sulfur dioxide. Add subscripts below to correctly identify the empirical formula of this compound (use this order of elements: CHSO)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1


Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2
You know the right answer?
A 33.153 mg sample of a chemical known to contain only carbon, hydrogen, sulfur, and oxygen is put i...
Questions

Mathematics, 14.04.2021 19:40



Mathematics, 14.04.2021 19:40

Chemistry, 14.04.2021 19:40



Mathematics, 14.04.2021 19:40



English, 14.04.2021 19:40



Mathematics, 14.04.2021 19:40




Mathematics, 14.04.2021 19:40