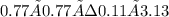A mixture containing initially 3.90 mole of NO(g) and 0.88 mole of CO2(g) was allowed to react in a flask of volume 1.00 L at a certain temperature according to the reaction CO2(g) + NO(g) ↔ CO(g) + NO2(g) At equilibrium 0.11 mole of CO2(g) was found present in the reaction mixture. Calculate the equilibrium constant Kc for the reaction at the temperature of the experiment.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 23.06.2019 07:00
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
You know the right answer?
A mixture containing initially 3.90 mole of NO(g) and 0.88 mole of CO2(g) was allowed to react in a...
Questions













Chemistry, 25.12.2019 03:31


Mathematics, 25.12.2019 03:31




Social Studies, 25.12.2019 03:31

Social Studies, 25.12.2019 03:31

![\frac{[CO] *[NO2]} {[CO2]*[NO]}](/tpl/images/0562/7676/90ab2.png)