
At a certain temperature this reaction follows second-order kinetics with a rate constant of Suppose a vessel contains at a concentration of . Calculate the concentration of in the vessel seconds later. You may assume no other reaction is important. Round your answer to significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 14:50
Write an equation to show action of positive and negative catalyst
Answers: 1
You know the right answer?
At a certain temperature this reaction follows second-order kinetics with a rate constant of Suppose...
Questions



Mathematics, 13.04.2021 04:40





Mathematics, 13.04.2021 04:40





Physics, 13.04.2021 04:40


Mathematics, 13.04.2021 04:40

Mathematics, 13.04.2021 04:40

Physics, 13.04.2021 04:40

Mathematics, 13.04.2021 04:40


Mathematics, 13.04.2021 04:40

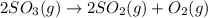
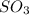 in the vessel after 0.240 seconds is 0.24 M
in the vessel after 0.240 seconds is 0.24 M![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0541/9921/5ea71.png)
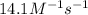
![[A]_o](/tpl/images/0541/9921/9caf5.png) = Initial concentration = 1.44 M
= Initial concentration = 1.44 M![14.1=\frac{1}{0.240}\left (\frac{1}{[A]}-\frac{1}{1.44}\right)](/tpl/images/0541/9921/8dfd8.png)
![[A]=0.245M](/tpl/images/0541/9921/0a4f3.png)


