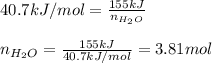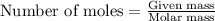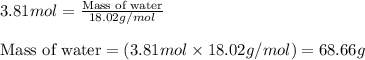Chemistry, 07.03.2020 05:07 moningersavannah
Calculate the mass of water (in g) that can be vaporized at its boiling point with 155 kJ of heat. • ΔHvap = 40.7 kJ/mol (at 100 °C) • 18.02 g H2O = 1 mol H2O

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2
You know the right answer?
Calculate the mass of water (in g) that can be vaporized at its boiling point with 155 kJ of heat. •...
Questions

Mathematics, 06.11.2021 08:10

Social Studies, 06.11.2021 08:10

English, 06.11.2021 08:20



English, 06.11.2021 08:20

Biology, 06.11.2021 08:20

Mathematics, 06.11.2021 08:20



Geography, 06.11.2021 08:20




Mathematics, 06.11.2021 08:20


English, 06.11.2021 08:20


Mathematics, 06.11.2021 08:20

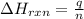
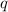 = amount of heat absorbed = 155 kJ
= amount of heat absorbed = 155 kJ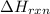 = enthalpy change of the reaction = 40.7 kJ/mol
= enthalpy change of the reaction = 40.7 kJ/mol