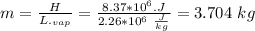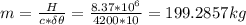Chemistry, 07.03.2020 03:56 damiangibson2
Assume that a daily diet of 2000 calories (i. e. 8.37 x 106 J) is converted completely to body heat.
a) How many g of water (as sweat) would need to evaporate to cool that person off?
b) If instead of evaporating water, the heat was used to raise the temperature of some water from 25.0 °C to 35.0 °C, how much water could be heated?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1
You know the right answer?
Assume that a daily diet of 2000 calories (i. e. 8.37 x 106 J) is converted completely to body heat....
Questions


Mathematics, 22.12.2019 09:31

Mathematics, 22.12.2019 09:31


Mathematics, 22.12.2019 09:31

Mathematics, 22.12.2019 09:31










Mathematics, 22.12.2019 09:31



Social Studies, 22.12.2019 09:31

Mathematics, 22.12.2019 09:31