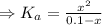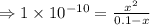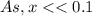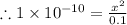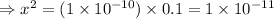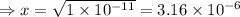Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3

Chemistry, 23.06.2019 10:00
The image shows the process of which is used in nuclear power plants. photo attached
Answers: 1
You know the right answer?
Bh+clo4- is a salt formed from the base b (kb = 1.00e-4) and perchloric acid. it dissociates into bh...
Questions


Biology, 24.11.2019 05:31




Mathematics, 24.11.2019 05:31


Mathematics, 24.11.2019 05:31

Mathematics, 24.11.2019 05:31


Mathematics, 24.11.2019 05:31



Mathematics, 24.11.2019 05:31

Mathematics, 24.11.2019 05:31

Mathematics, 24.11.2019 05:31


Mathematics, 24.11.2019 05:31


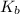 = 1 × 10⁻⁴, Concentration of salt: BH⁺ClO₄⁻ = 0.1 M
= 1 × 10⁻⁴, Concentration of salt: BH⁺ClO₄⁻ = 0.1 M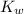 = 1 × 10⁻¹⁴
= 1 × 10⁻¹⁴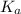 ) for the weak acid (BH⁺) can be calculated by the equation:
) for the weak acid (BH⁺) can be calculated by the equation: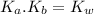
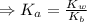
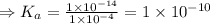
![K_{a} = \frac{\left [B \right ] \left [H_{3}O^{+}\right ]}{\left [BH^{+} \right ]} = \frac{(x)(x)}{(0.1 - x)} = \frac{x^{2}}{0.1 - x}](/tpl/images/0434/5359/b792d.png)