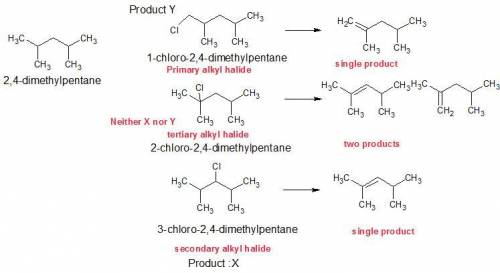
Compounds x and y are both c7h15cl products formed in the radical chlorination of 2,4-dimethylpentane. base-promoted e2 elimination of x and y gives, in each case, a single c7h14 alkene. both x and y undergo an sn2 reaction with sodium iodide in acetone solution to give c7h15i products; in this reaction y reacts faster than x. what is the structure of x?

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1
You know the right answer?
Compounds x and y are both c7h15cl products formed in the radical chlorination of 2,4-dimethylpentan...
Questions

Social Studies, 26.03.2020 23:24


Spanish, 26.03.2020 23:24

Mathematics, 26.03.2020 23:24



English, 26.03.2020 23:24

Mathematics, 26.03.2020 23:24



Biology, 26.03.2020 23:25

Chemistry, 26.03.2020 23:25


Advanced Placement (AP), 26.03.2020 23:25

Social Studies, 26.03.2020 23:25