
Chemistry, 21.10.2019 22:00 99keevintaylor012
According to the quantum-mechanical model for the hydrogen atom, which of the following electron transitions would produce light with the longer wavelength: 2p→1s or 3p→1s?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3
You know the right answer?
According to the quantum-mechanical model for the hydrogen atom, which of the following electron tra...
Questions


Chemistry, 18.12.2019 20:31





Chemistry, 18.12.2019 20:31

Mathematics, 18.12.2019 20:31












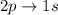
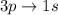 is greater as compared to
is greater as compared to 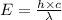
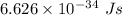
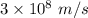
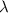 is the wavelength
is the wavelength 

