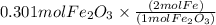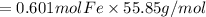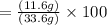Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3
You know the right answer?
Combining 0.301 mol fe2o3
with excess carbon produced 11.6 g fe.
fe2o3+3c⟶2fe+3co<...
with excess carbon produced 11.6 g fe.
fe2o3+3c⟶2fe+3co<...
Questions

Biology, 23.12.2020 08:50



Health, 23.12.2020 08:50

Mathematics, 23.12.2020 08:50


English, 23.12.2020 09:00


Mathematics, 23.12.2020 09:00

Mathematics, 23.12.2020 09:00

English, 23.12.2020 09:00

Mathematics, 23.12.2020 09:00

Mathematics, 23.12.2020 09:00

Mathematics, 23.12.2020 09:00


Mathematics, 23.12.2020 09:00

Mathematics, 23.12.2020 09:00


English, 23.12.2020 09:00

English, 23.12.2020 09:00

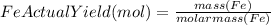
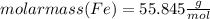
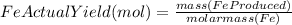
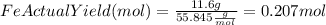
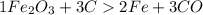
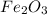 to moles Fe and moles Fe to mass Fe
to moles Fe and moles Fe to mass Fe 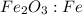 is 1 : 2
is 1 : 2