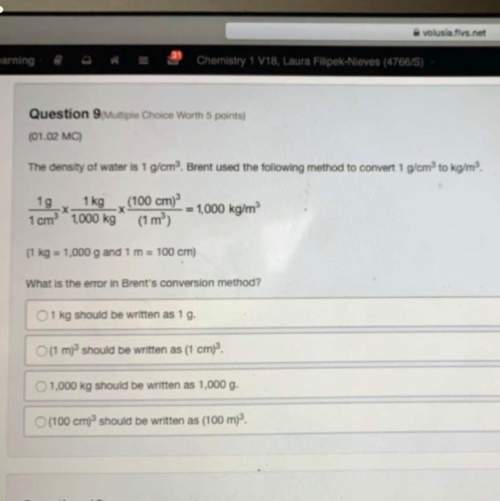
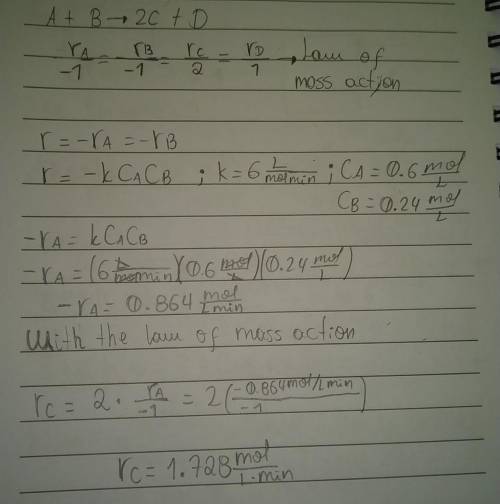Chemistry, 24.09.2019 20:00 soulspiritsa
What is the instantaneous rate of formation of product c given the following information: a. stoichiometric equation a+ b2c+ d b. applicable rate equation is r.-k"ca"cb c. the rate constant is 6.0 liters/(mole-minute) d. the current concentrations of a and b species are ca 0.6 moles/liter and ca 0.24 moles/liter

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 11:30
Which part of the healthcare system could best explain how a pharmaceutical drug works
Answers: 2
You know the right answer?
What is the instantaneous rate of formation of product c given the following information: a. stoich...
Questions

Mathematics, 03.08.2019 04:30




Physics, 03.08.2019 04:30



Mathematics, 03.08.2019 04:30


Social Studies, 03.08.2019 04:30






English, 03.08.2019 04:30


English, 03.08.2019 04:30