
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o2) are carbon dioxide (co2) and water (h2o).
a mass of 16.74 g for an unknown fuel was combusted in a reaction vessel containing an unknown amount of oxygen. at the end of the reaction, there still remained 16.70 g of the fuel as well as 0.0654 g of water and 0.1198 g of carbon dioxide. the oxygen was completely consumed during the reaction.
how many molecules of oxygen gas were initially present in the reaction vessel?
me, i will be very . the topic is limiting reagents and theoretical yields.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:30
4nh3+5o2--> 4no+6h20what is the total number of moles of h2o produced when 12 mole of nh3 is completely consumed?
Answers: 3

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1
You know the right answer?
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o...
Questions

Mathematics, 01.07.2019 06:00

Mathematics, 01.07.2019 06:00


Mathematics, 01.07.2019 06:00


Mathematics, 01.07.2019 06:00



Biology, 01.07.2019 06:00


Mathematics, 01.07.2019 06:00


Chemistry, 01.07.2019 06:00

Mathematics, 01.07.2019 06:00

Advanced Placement (AP), 01.07.2019 06:00

Biology, 01.07.2019 06:00




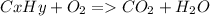
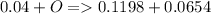
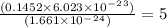 molecules
molecules


