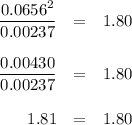Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(=1.80 at 250 ∘c a 0.197 mol sample of pcl5(g) is injected into an empty 2.90 l reaction vessel held at 250 ∘c. calculate the concentrations of pcl5(g) and pcl3(g) at equilibrium.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1

Chemistry, 23.06.2019 09:30
If the solubility of a gas in water is 1.22g/2.75 atm, what is it’s solubility (in g/l) at 1.0 atm
Answers: 1
You know the right answer?
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(=1.80...
Questions

Social Studies, 22.03.2021 17:30

History, 22.03.2021 17:30




Advanced Placement (AP), 22.03.2021 17:30

Biology, 22.03.2021 17:30



Mathematics, 22.03.2021 17:30

Biology, 22.03.2021 17:30


Computers and Technology, 22.03.2021 17:30

Mathematics, 22.03.2021 17:30



Spanish, 22.03.2021 17:30


Mathematics, 22.03.2021 17:30

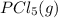 is 0.0655 M and
is 0.0655 M and 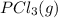 is 0.00240 M at equilibrium.
is 0.00240 M at equilibrium.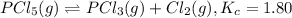
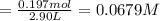

![[PCl_3]=x](/tpl/images/0128/9812/3c44a.png)
![[Cl_2] = x](/tpl/images/0128/9812/a7a23.png)
![=[PCl_5]= (0.0697- x)](/tpl/images/0128/9812/ead0f.png)
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}\\\\1.80=\frac{x\times x}{(0.0679-x)}\\\\x = 0.0655](/tpl/images/0128/9812/79ef0.png)
![=[PCl_5]= (0.0679- x) = (0.0679 -0.0655 )M=0.00240 M](/tpl/images/0128/9812/ac2f8.png)
![= [Cl_2] = x = 0.0655 M](/tpl/images/0128/9812/6faf5.png)
![\text{[PCl$_{5}$]} = \dfrac{\text{0.197 mol}}{\text{2.90 L}} = \text{0.067 93 mol/L}\\\\](/tpl/images/0128/9812/66356.png)
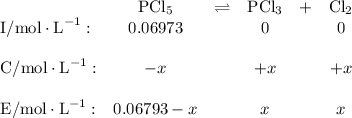
![K_{\text{c}} = \dfrac{\text{[PCl$_3$][Cl$_2$]}}{\text{[PCl$_5$]}} = \dfrac{x^{2}}{0.06793-x} = 1.80\\\\\begin{array}{rcl}\\x^{2}& = & 1.80(0.06793 - x)\\x^{2& = & 0.1223 - 1.80x\\x^{2} + 1.80x - 0.1223& = & 0\\x & = & \mathbf{0.0656}\\\end{array}](/tpl/images/0128/9812/cf1bb.png)