
Chemistry, 30.11.2019 08:31 SuperWoman9172
Carbon disulfide burns in oxygen to yield car- bon dioxide and sulfur dioxide according to the chemical equation cs2(l) 3 o2(g) −→ co2(g) 2 so2(g). if 0.91 mol of cs2 is combined with 1.52 mol of o2, identify the limiting reactant.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

You know the right answer?
Carbon disulfide burns in oxygen to yield car- bon dioxide and sulfur dioxide according to the chemi...
Questions

Geography, 22.08.2019 12:50

Mathematics, 22.08.2019 12:50

Biology, 22.08.2019 12:50

English, 22.08.2019 12:50



History, 22.08.2019 12:50


English, 22.08.2019 12:50



Computers and Technology, 22.08.2019 12:50


Biology, 22.08.2019 12:50

Mathematics, 22.08.2019 12:50



Mathematics, 22.08.2019 12:50


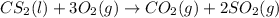
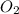 reacts with 1 mole of
reacts with 1 mole of 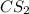
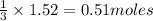 of
of 

