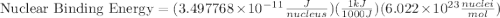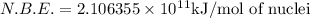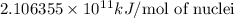Chemistry, 10.07.2019 12:30 janicemaxwell123
How much energy must be supplied to break a single aluminum-27 nucleus into separated protons and neutrons if an aluminum-27 atom has a mass of 26.9815386 amu? (the mass of an electron is 5.485799×10−4 amu, the mass of a proton is 1.0072765 amu, and the mass of a neutron is 1.0086649 amu.) express your answer using six significant figures. g?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3


Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
How much energy must be supplied to break a single aluminum-27 nucleus into separated protons and ne...
Questions







Mathematics, 20.07.2019 00:30


Chemistry, 20.07.2019 00:30


History, 20.07.2019 00:30

Biology, 20.07.2019 00:30

History, 20.07.2019 00:30

Biology, 20.07.2019 00:30


Chemistry, 20.07.2019 00:30



Social Studies, 20.07.2019 00:30

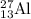 nucleus having 13 protons as it is visible from atomic number and number of neutrons is 14 which is calculated from
nucleus having 13 protons as it is visible from atomic number and number of neutrons is 14 which is calculated from 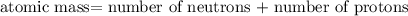 .
. 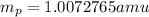 and
and 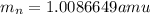 , we can calculate the mass of the isotope.
, we can calculate the mass of the isotope.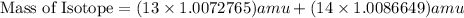
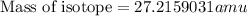
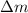 = mass of isotope - atomic mass.
= mass of isotope - atomic mass.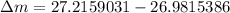
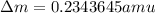
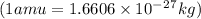
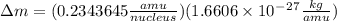
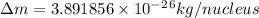
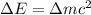
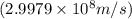
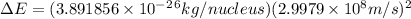
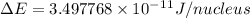
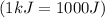 and converting individual particles into moles, we need to multiply it by avagadro's number that is
and converting individual particles into moles, we need to multiply it by avagadro's number that is 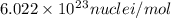 .
.