
Chemistry, 12.07.2019 12:00 jholland03
Mercury (a. k.a. quicksilver) is a metallic element and a liquid at room temperature. calculate mercury\'s density if a sample of mercury is found to have a mass of 589.0 g and a volume of 43.86 ml.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
Mercury (a. k.a. quicksilver) is a metallic element and a liquid at room temperature. calculate merc...
Questions




















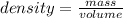 -(1)
-(1)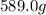 (given)
(given)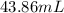 (given)
(given)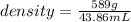
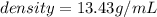
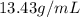 .
.

