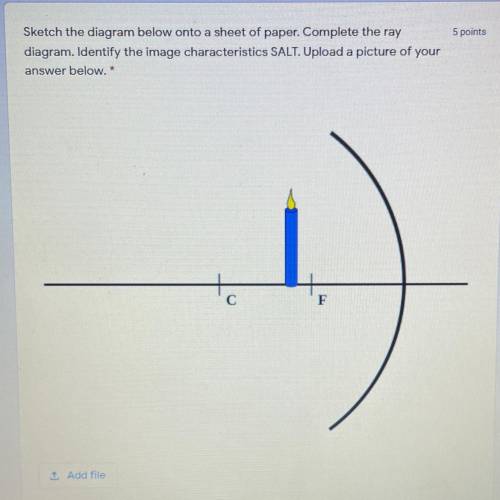
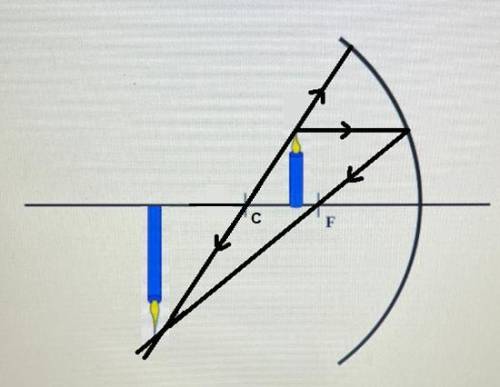
Optics Quiz
can somebody please help me!
...

Answers: 3
Another question on Physics

Physics, 22.06.2019 21:00
Aflask with vinegar in it has a mass of 160 grams. a balloon with baking soda in it has a mass of 40 grams. the balloon is attached to the flask to seal the opening and the vinegar and baking soda mixes. the balloon inflates to a large volume. what will the total mass of the balloon and flask be after the balloon inflates? explain. a) less than 200 grams because the solid baking soda disappears. b) 200 grams, because all the atoms remain in the balloon or flask. c) more than 200 grams because the size of the balloon is so much larger. d) less than 200 grams because gases such as the one in the balloon are lighter than solids and liquids.
Answers: 1

Physics, 22.06.2019 21:10
Which of the following is a measure of the amount of light a star directly emits? a. luminosity b. intensity c. wavelength d. brightness
Answers: 2

Physics, 22.06.2019 21:30
Calculate the minimum energy required to remove one proton from the nucleus 126c. this is called the proton-removal energy. (hint: find the difference between the mass of a 126c nucleus and the mass of a proton plus the mass of the nucleus formed when a proton is removed from 126c.) express your answer with the appropriate units. emin e m i n = nothing nothing request answer part b how does the proton-removal energy for 126c compare to the binding energy per nucleon for 126c, calculated using eb=(zmh+nmn−azm)c2?
Answers: 1

Physics, 23.06.2019 09:00
Give an example of a collision in real life. use the law of conservation of energy to describe the transfer of momentum. be sure and discuss the momentum before and after the collision occurs. you will need at least 3 sentences to thoroughly answer this question.
Answers: 3
You know the right answer?
Questions



















Mathematics, 10.07.2019 03:20