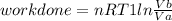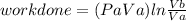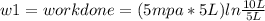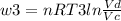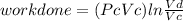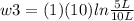Physics, 19.02.2021 17:00 bkimswift7
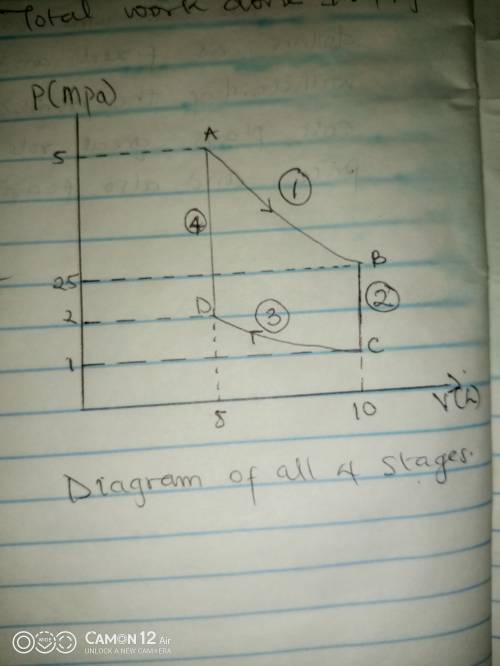
Thermodynamic Processes Two moles of a monatomic ideal gas at (5 MPa, 5 L) is expanded isothermally until the volume is doubled (step 1). Then it is cooled isochorically until the pressure is 1 MPa (step 2). The temperature drops in this process. The gas is now compressed isothermally until its volume is back to 5 L, but its pressure is now 2 MPa (step 3). Finally, the gas is heated isochorically to return to the initial state (step 4). (a) Draw the four processes in the pV plane. (b) Find the total work done by the gas.

Answers: 1
Another question on Physics

Physics, 22.06.2019 07:30
Tall pacific coast redwood trees can reach heights of about 100 m. if air drag is negligibly small, how fast is a sequola cone moving when it reaches the ground f it dropped from the top of a 100 m tree?
Answers: 1

Physics, 22.06.2019 10:30
You are given two vectors a⃗ =−3.00ι^ 5.00j^ and b⃗ =5.00ι^ 2.00j^. let the counterclockwise angles be positive.
Answers: 3

Physics, 22.06.2019 13:10
Most short-period comets do not have randomly oriented orbits because
Answers: 2

Physics, 22.06.2019 13:30
The period of a pendulum varies directly as the square root of the length of the pendulum and inversely as the square root of the acceleration due to gravity. find the period when the length is 144 cm and the acceleration due to gravity is 980 cm per second squared, if the period is 7pi seconds when the length is 289 cm and the acceleration due to gravity is 980 cm per second squared.
Answers: 2
You know the right answer?
Thermodynamic Processes
Two moles of a monatomic ideal gas at (5 MPa, 5 L) is expanded isothermally...
Questions

History, 30.09.2020 01:01






Mathematics, 30.09.2020 01:01

Mathematics, 30.09.2020 01:01

Mathematics, 30.09.2020 01:01



History, 30.09.2020 01:01

History, 30.09.2020 01:01

Mathematics, 30.09.2020 01:01


Mathematics, 30.09.2020 01:01

Mathematics, 30.09.2020 01:01


Social Studies, 30.09.2020 01:01