
Physics, 08.10.2020 01:01 ashtyndowdle
2. The visible region of the hydrogen spectrum results from relaxation of electrons from excited states to energy level 2 (n1). Use the Rydberg equation and your measured wavelengths to determine the energy transitions associated with each of your observed wavelengths for hydrogen. In other words, calculate the excited state energy level (n2) for each of your observed wavelengths for hydrogen. n has integer values; so, calculate it first with appropriate significant digits, then round it to an integer. Use the key to show your work for at least one calculation. Must show energy levels for each hydrogen wavelength.

Answers: 2
Another question on Physics

Physics, 22.06.2019 10:30
An insulated 40 ft^3 rigid tank contains air at 50 psia and 120°f. a valve connected to the tank is now opened, and air is allowed to escape until the pressure inside drops to 25 psia. the air temperature during this process is kept constant by an electric resistance heater placed in the tank. determine the electrical work done during this process.
Answers: 2

Physics, 22.06.2019 14:00
How much energy must a refrigerator absorb from 225 g of water so that the temperature of the water will drop from 35°c to 5°c
Answers: 3

Physics, 22.06.2019 14:30
Which compound is held together by the electrostatic force between two ions? a. co2 b. cci4 c. h2s d. mgf2
Answers: 1

Physics, 22.06.2019 14:40
Sort the images based on whether there's a force of attraction or repulsion between the magnets shown. attraction repulsion
Answers: 2
You know the right answer?
2. The visible region of the hydrogen spectrum results from relaxation of electrons from excited sta...
Questions


Arts, 22.10.2019 16:00

Physics, 22.10.2019 16:00


Biology, 22.10.2019 16:00



History, 22.10.2019 16:00

Social Studies, 22.10.2019 16:00

English, 22.10.2019 16:00



Mathematics, 22.10.2019 16:00

Social Studies, 22.10.2019 16:00

Mathematics, 22.10.2019 16:00

Mathematics, 22.10.2019 16:00


Mathematics, 22.10.2019 16:00


Mathematics, 22.10.2019 16:00

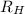 (1/2² - 1 / n²)
(1/2² - 1 / n²)


