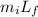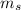Physics, 22.04.2020 03:55 camiserjai1832
What mass of steam at 100°C must be mixed with 490 g of ice at its melting point, in a thermally insulated container, to produce liquid water at 89.0°C? The specific heat of water is 4186 J/kg·K. The latent heat of fusion is 333 kJ/kg, and the latent heat of vaporization is 2256 kJ/kg.

Answers: 1
Another question on Physics

Physics, 22.06.2019 01:30
Kate is working on a project in her tech education class. she plans to assemble a fan motor. which form of energy does the motor convert most of its electric energy into? a. light energy b. motion energy c. sound energy d. thermal energy
Answers: 1


Physics, 22.06.2019 18:00
Which statement is the best definition of an atom? a) anything that has mass and occupies space. b) the smallest particle that has the properties of an element. c) a substance that cannot be broken down chemically into simpler substances. eliminate d) the smallest unit of a substance that exhibits all of the properties characteristic of that substance.
Answers: 2

Physics, 22.06.2019 19:20
On a hot saturday morning while people are working inside, the air conditioner keeps the temperature inside the building at 22degreesc. at noon the air conditioner is turned off, and the people go home. the temperature outside is a constant 33degreesc for the rest of the afternoon. if the time constant for the building is 4 hr, what will be the temperature inside the building at 4 : 00 font size decreased by 2 upper p . font size decreased by 2 upper m .? at 6 : 00 font size decreased by 2 upper p . font size decreased by 2 upper m .? when will the temperature inside the building reach 24degreesc? 324281
Answers: 3
You know the right answer?
What mass of steam at 100°C must be mixed with 490 g of ice at its melting point, in a thermally ins...
Questions


Social Studies, 03.03.2020 20:54


Geography, 03.03.2020 20:54

English, 03.03.2020 20:54

Mathematics, 03.03.2020 20:54

Mathematics, 03.03.2020 20:54





English, 03.03.2020 20:54

Mathematics, 03.03.2020 20:54



History, 03.03.2020 20:54

Mathematics, 03.03.2020 20:54

History, 03.03.2020 20:54


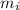 = 490 g = 0.49 kg
= 490 g = 0.49 kg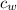 = 4186 J/kg.K = 4.186 kJ/kg.K
= 4186 J/kg.K = 4.186 kJ/kg.K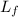 = 333 kJ/kg
= 333 kJ/kg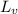 = 2256 kJ/kg
= 2256 kJ/kg