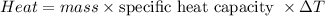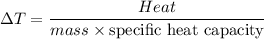Physics, 11.03.2020 02:50 pricilaxo9523
Five-gram samples of copper and aluminum are at room temperature. Both receive equal amounts of energy due to heat flow. The specific heat capacity of copper is 0.09 cal/g°C, and the specific heat capacity of aluminum is 0.22 cal/g°C. Which of the following statements is true?
a. The temperature of each sample increases by the same amount.
b. The aluminum will get hotter than the copper.
c. The copper will get hotter than the aluminum.
d. The temperature of each sample decreases by the same amount

Answers: 2
Another question on Physics

Physics, 22.06.2019 05:30
An object weighs 40n in air, weighs 20n when submerged in water and 30n when submerged in a liquid of unknown density. what is the density of the liquid?
Answers: 2

Physics, 22.06.2019 10:00
Asap and show ! a 14 kg rock starting from rest free falls through a distance of 5.0 m with no air resistance. find the momentum change of the rock caused by its fall and the resulting change in the magnitude of earths velocity. earth mass is 6.0 * 10^24 kg. show your work assuming the rock earth system is closed.
Answers: 2

Physics, 22.06.2019 14:30
Two carts, one of mass 2m and one of mass m, approach each other with the same speed, v. when the carts collide, they hook together. assume that positive momentum is to the right. which graph best represents the momentum of both carts over time, before and after the collision?
Answers: 3

Physics, 22.06.2019 15:00
10 points! will mark brainiest! in a heat engine if 1,000 j of heat enters the system and the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2,000 j 1: write the equation 2: list out your known variables 3: plug the numbers into the equations 4: solve 5: write your solution statement that includes initial energy and final energy added you so much!
Answers: 3
You know the right answer?
Five-gram samples of copper and aluminum are at room temperature. Both receive equal amounts of ener...
Questions


Mathematics, 23.06.2019 21:30

History, 23.06.2019 21:30


Mathematics, 23.06.2019 21:30


Chemistry, 23.06.2019 21:30

Mathematics, 23.06.2019 21:30


Mathematics, 23.06.2019 21:30

Mathematics, 23.06.2019 21:30

History, 23.06.2019 21:30

History, 23.06.2019 21:30





Chemistry, 23.06.2019 21:40


Mathematics, 23.06.2019 21:40