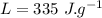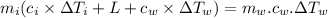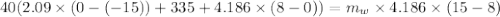A40.0-g block of ice at -15.00°c is dropped into a calorimeter (of negligible heat capacity) containing water at 15.00°c.
when equilibrium is reached, the final temperature is 8.00°c.
how much water did the calorimeter contain initially?
the specific heat of ice is 2090 j/kg • k, that of water is 4186 j/kg • k, and the latent heat of fusion of water is 33.5 × 104 j/kg.

Answers: 1
Another question on Physics

Physics, 22.06.2019 10:00
Abookcase has a mass of 38 kg and the coefficient of friction between it and the floor is 0.82 what is the maximum force of friction between the bookcase and the floor a 372n b 305n c 412n d 449n
Answers: 1

Physics, 22.06.2019 12:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 2

Physics, 22.06.2019 14:10
Amachinist turns the power on to a grinding wheel, at rest, at time t = 0 s. the wheel accelerates uniformly for 10 s and reaches the operating angular velocity of 96 rad/s. the wheel is run at that angular velocity for 40 s and then power is shut off. the wheel slows down uniformly at 1.5 rad/s2 until the wheel stops. in this situation, the time interval of deceleration is closest to:
Answers: 3

You know the right answer?
A40.0-g block of ice at -15.00°c is dropped into a calorimeter (of negligible heat capacity) contain...
Questions

Mathematics, 19.08.2019 03:30

Mathematics, 19.08.2019 03:30


Mathematics, 19.08.2019 03:30

Chemistry, 19.08.2019 03:30

History, 19.08.2019 03:30

Mathematics, 19.08.2019 03:30

Mathematics, 19.08.2019 03:30

Biology, 19.08.2019 03:30

History, 19.08.2019 03:30

Biology, 19.08.2019 03:30

Mathematics, 19.08.2019 03:30

Social Studies, 19.08.2019 03:30

Mathematics, 19.08.2019 03:30


Physics, 19.08.2019 03:30




History, 19.08.2019 03:30

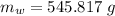
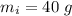 initial temperature of ice block,
initial temperature of ice block,  initial temperature of water,
initial temperature of water,  final temperature of mixture,
final temperature of mixture, 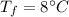 specific heat of ice,
specific heat of ice, 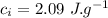 specific heat of water,
specific heat of water, 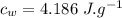 Latent heat of fusion of water,
Latent heat of fusion of water,