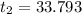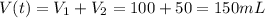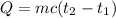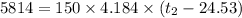Physics, 08.10.2019 04:00 Kingdcn6261
A100.0 ml sample of 1.020 m hcl is mixed with a 50.0 ml sample of 2.040 m naoh in a styrofoam cup. if both solutions were initially at 24.53°c, and the enthalpy of the neutralization reaction is −57 kj/mole of h2o formed, what is the final temperature of the mixture? assume that the solution has a density of 1.00 g/ml and a specific heat of 4.184 j/g°c, and that the styrofoam cup has an insignificant heat capacity.

Answers: 2
Another question on Physics

Physics, 22.06.2019 04:00
However, had it been a real sound, the sound's pitch would have been increased by the doppler effect, since the falcon was moving the source of the sound. perpendicular to away from towards at the same speed as
Answers: 1

Physics, 22.06.2019 16:10
Amass weighing 24 pounds, attached to the end of a spring, stretches it 4 inches. initially, the mass is released from rest from a point 8 inches below the equilibrium position. find the equation of motion. (use g = 32 ft/s2 for the acceleration due to gravity.)
Answers: 1

Physics, 22.06.2019 18:30
En un laboratorio, nakisha mezcla una solución de hidróxido de sodio con un indicador llamado fenolftaleína. cuando se combinan, crean una solución de color rosa. nakisha se pregunta si la mezcla de otras soluciones con fenolftaleína también creará este color rosa. ? cómo podría nakisha utilizar el proceso de investigación científica para determinar si la mezcla de otras soluciones con fenolftaleína también creará un color rosa? marque las que correspondan.
Answers: 2

Physics, 23.06.2019 03:50
For most stars, what does a higher temperature tend to to with? a lower luminosity a higher luminositya red color none of the above
Answers: 1
You know the right answer?
A100.0 ml sample of 1.020 m hcl is mixed with a 50.0 ml sample of 2.040 m naoh in a styrofoam cup. i...
Questions


Physics, 25.06.2021 16:30




Geography, 25.06.2021 16:30

Mathematics, 25.06.2021 16:30


History, 25.06.2021 16:30



Social Studies, 25.06.2021 16:30

Chemistry, 25.06.2021 16:30

Mathematics, 25.06.2021 16:30

Biology, 25.06.2021 16:30



Chemistry, 25.06.2021 16:30