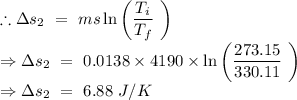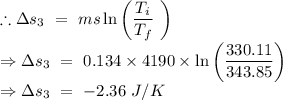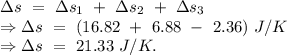Physics, 14.09.2019 02:30 kelseychristian24
An insulated thermos contains 134 g of water at 70.7°c. you put in a 13.8 g ice cube at 0.00°c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg*k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?

Answers: 2
Another question on Physics

Physics, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Physics, 22.06.2019 15:00
Consider a uniformly charged ring in the xy plane, centered at the origin. the ring has radius a and positive charge qdistributed evenly along its circumference. a)what is the direction of the electric field at any point on the z axis? . b)what is the magnitude of the electric field along the positive z axis? use k in your answer, where k=14πϵ0. d)the ball will oscillate along the z axis between z=d and z=−d in simple harmonic motion. what will be the angular frequency ω of these oscillations? use the approximation d≪a to simplify your calculation; that is, assume that d2+a2≈a2. express your answer in terms of given charges, dimensions, and constants
Answers: 2


Physics, 22.06.2019 18:00
Atank is filled with an ideal gas at 400 k and pressure of 1.00 atm . part a the tank is heated until the pressure of the gas in the tank doubles. what is the temperature of the gas?
Answers: 3
You know the right answer?
An insulated thermos contains 134 g of water at 70.7°c. you put in a 13.8 g ice cube at 0.00°c to fo...
Questions

Biology, 28.08.2019 02:30


History, 28.08.2019 02:30

Mathematics, 28.08.2019 02:30

Mathematics, 28.08.2019 02:30

Social Studies, 28.08.2019 02:30

Mathematics, 28.08.2019 02:30




English, 28.08.2019 02:30


Mathematics, 28.08.2019 02:30

English, 28.08.2019 02:30




Computers and Technology, 28.08.2019 02:30

Mathematics, 28.08.2019 02:30

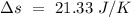
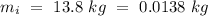 Temperature of the ice =
Temperature of the ice = 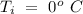 Mass of the original water =
Mass of the original water = 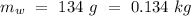 Temperature of the original water =
Temperature of the original water = 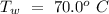 Specific heat of water =
Specific heat of water = 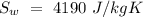 Latent heat of fusion of ice =
Latent heat of fusion of ice = 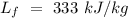
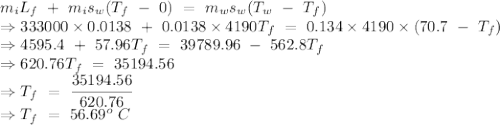
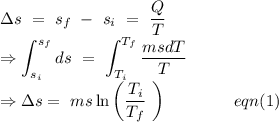
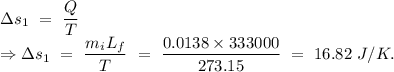
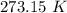 to water 330.11 K water.
to water 330.11 K water.