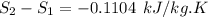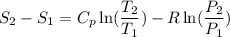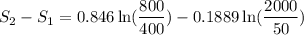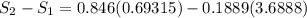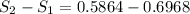Engineering, 14.07.2020 14:01 sandram74691
Carbon dioxide initially at 50 kPa, 400 K, undergoes a process in a closed system until its pressure and temperature are 2 MPa and 800 K, respectively. Assuming an ideal gas behaviour, find the entropy change of the carbon dioxide by assuming that the specific heats are constant. For the gas, take Cp = 0.846 kJ/kg. K and R = 0.1889 kJ/kg. K

Answers: 3
Another question on Engineering

Engineering, 04.07.2019 08:10
Which of the following is an easy way to remember the modified “x” tire rotation? a. nondrive wheels straight, cross the drive wheels b. drive wheels straight, cross the nondrive wheels c. drive wheels crossed, nondrive wheels straight d. drive wheels crossed, nondrive wheels crossed
Answers: 1

Engineering, 04.07.2019 16:10
An electrical motor raises a 50kg load at a construct velencity .calculate the power of the motor, if it takes 40sec to raise the load through a height of 24m(take g =9.8n/g)
Answers: 2

Engineering, 04.07.2019 18:10
Coiled springs ought to be very strong and stiff. si3n4 is a strong, stiff material. would you select this material for a spring? explain.
Answers: 2

Engineering, 04.07.2019 18:10
During a steady flow process, the change of energy with respect to time is zero. a)- true b)- false
Answers: 2
You know the right answer?
Carbon dioxide initially at 50 kPa, 400 K, undergoes a process in a closed system until its pressure...
Questions





Mathematics, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00




Mathematics, 18.11.2020 01:00

History, 18.11.2020 01:00


Computers and Technology, 18.11.2020 01:00



History, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00