
Engineering, 14.02.2020 17:26 sarinawhitaker
Using the results of the Arrhenius analysis (Ea=93.1kJ/molEa=93.1kJ/mol and A=4.36×1011M⋅s−1A=4.36×1011M⋅s−1), predict the rate constant at 332 KK . Express the rate constant in liters per mole-second to three significant figures.

Answers: 2
Another question on Engineering

Engineering, 04.07.2019 18:20
A2-m rigid tank initially contains saturated water vapor at 100 kpa. the tank is connected to a supply line through a valve. steam is flowing in the supply line at 600 kpa and 300 c. the valve is opened, and steam is allowed to enter the tank until the pressure in the tank reaches the line pressure, at which point the valve is closed. a thermometer placed in the tank indicates that the temperature at the final state is 200°c. determine (a) the mass of steam that has entered the tank (b) the amount of heat transfer.
Answers: 3

Engineering, 04.07.2019 18:20
Water vapor initially at 10 bar and 400 °c is contained within a piston-cylinder assembly. the water lost heat to the surrounding according to isochoric (iso-volumetric) process until its temperature is 150 °c. the water is then condensed isothermally to saturated liquid. for the water as a system, calculate the work in kj/kg
Answers: 2

Engineering, 04.07.2019 19:10
What is a monomer? how do they form a ploymer from the view point of chemical bonding?
Answers: 1

Engineering, 04.07.2019 19:10
Arigid tank contains 10 kg of air at 137 kpa (abs) and 21°c. more air is added to the tank until the pressure and temperature rise to 242 kpa (abs) and 32°c, respectively. determine the amount of air added to the tank. [r-0.287 kj/kg k]
Answers: 3
You know the right answer?
Using the results of the Arrhenius analysis (Ea=93.1kJ/molEa=93.1kJ/mol and A=4.36×1011M⋅s−1A=4.36×1...
Questions


Mathematics, 20.03.2021 04:20




Mathematics, 20.03.2021 04:20

Health, 20.03.2021 04:20

Mathematics, 20.03.2021 04:20




Biology, 20.03.2021 04:20

Mathematics, 20.03.2021 04:20

Chemistry, 20.03.2021 04:20

Mathematics, 20.03.2021 04:20

Computers and Technology, 20.03.2021 04:20


Mathematics, 20.03.2021 04:20

Social Studies, 20.03.2021 04:20

Mathematics, 20.03.2021 04:20

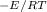 ------------------- euqation (1)
------------------- euqation (1)![^{[(-93.1*10^3)(J/mol)]/[(8.314)(J/mol.K)(332K)}](/tpl/images/0511/5538/b5cb2.png)


