Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 23.06.2019 21:00
In a buffer system of hf and its salt, naf, the f- neutralizes added base the hf is not necessary the hf neutralizes added base the hf neutralizes added acid the f- neutralizes added h2o
Answers: 3
You know the right answer?
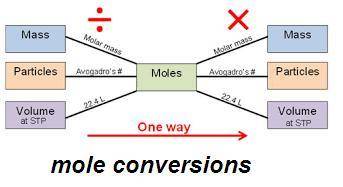
How many grams of calcium chloride are needed to produce 1.50 g of potassium chloride? cacl2(aq) +...
Questions







Mathematics, 20.10.2020 18:01







Mathematics, 20.10.2020 18:01

History, 20.10.2020 18:01

Social Studies, 20.10.2020 18:01

History, 20.10.2020 18:01



Mathematics, 20.10.2020 18:01

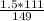 X g of CaCl₂ = 1.12 g
X g of CaCl₂ = 1.12 g