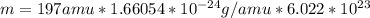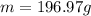Chemistry, 22.07.2019 00:00 christabell0303
Question 3 given that au-197 is the only natural isotope of gold, what is the mass of one au atom and the mass of avogadro's number of au atoms, respectively? 196.97 g and 196.97 amu 197 amu and 197 g 197 g and 197 amu 196.97 amu and 196.97 g none of the above

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
Question 3 given that au-197 is the only natural isotope of gold, what is the mass of one au atom an...
Questions

Mathematics, 02.10.2019 22:00

Chemistry, 02.10.2019 22:00


Mathematics, 02.10.2019 22:00

History, 02.10.2019 22:00



English, 02.10.2019 22:00

English, 02.10.2019 22:00


Mathematics, 02.10.2019 22:00


Mathematics, 02.10.2019 22:00

Mathematics, 02.10.2019 22:00

Mathematics, 02.10.2019 22:00

Social Studies, 02.10.2019 22:00

Mathematics, 02.10.2019 22:00

English, 02.10.2019 22:00

Biology, 02.10.2019 22:00

Mathematics, 02.10.2019 22:00