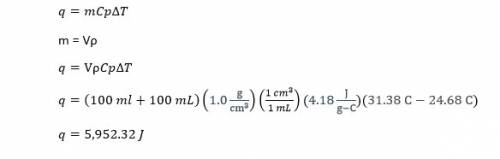In a coffee-cup calorimeter, 100.0 ml of 1.0 m naoh and 100.0 ml of 1.0 m hcl are mixed. both solutions were originally at 24.68c. after the reaction, the final temperature is 31.38c. assuming that all the solutions have a density of 1.0 g/cm3 and a specific heat capacity of 4.18 j/8c ? g, calculate the enthalpy change for the neutralization of hcl by naoh

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
In a coffee-cup calorimeter, 100.0 ml of 1.0 m naoh and 100.0 ml of 1.0 m hcl are mixed. both soluti...
Questions


Chemistry, 16.09.2019 07:10



Social Studies, 16.09.2019 07:10



Mathematics, 16.09.2019 07:10

Geography, 16.09.2019 07:10

Medicine, 16.09.2019 07:10

Mathematics, 16.09.2019 07:10

Computers and Technology, 16.09.2019 07:10


History, 16.09.2019 07:10




Mathematics, 16.09.2019 07:10

Biology, 16.09.2019 07:10